Fellows’ corner

Dr Shefali Sharma, Rheumatology, PGIMER, Chandigarh
A forgotten mode…
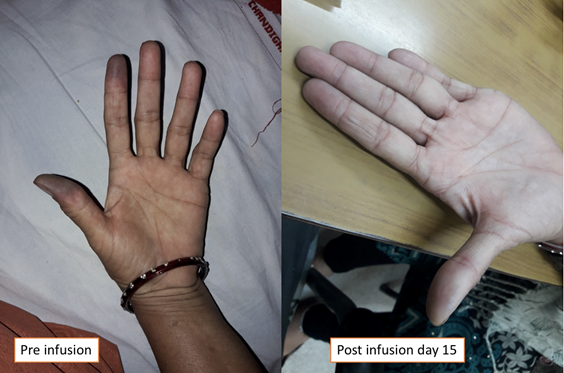
A 45-year-old female non-diabetic, non-hypertensive, hypothyroid patient on 75 µg of levothyroxine supplementation for the past 5 years presented to the Rheumatology OPD of PGIMER Chandigarh with complaints of gradually progressive blackish discoloration of tip of the left thumb and index finger for the past 20 days. It was associated with severe pain, disturbing her sleep, increasing on exposure to cold temperature. She had a similar episode during the previous winter, which had resolved with conservative management. She had no history of joint pains, skin tightening, oral ulcer, dysphagia, skin rash, alopecia, dryness of eyes or mouth, or family history of similar illness. She was started on conservative management in the form of vasodilatory therapy elsewhere, but her symptoms progressed to involve the distal phalanges of her thumb and index finger. In view of progression of symptoms, she was admitted to the hospital for Inj Alprostadil (PGE1) infusion. On clinical examination, all the peripheral pulses were equally palpable, and there was no blood pressure difference. Investigations including procoagulant and ANA (by IIF in Hep2) work-ups were negative. Doppler of the concerned vessel showed biphasic flow in the radial artery of the left hand. She was started on Inj. alprostadil 60 mcg/day for 5 consecutive days, as per product guideline. There was a significant reduction of pain as per visual analog scale (VAS) and patient global assessment after starting the infusion. There was no adverse event during the infusion. The blackish discoloration on the fingers started regressing. There was only mild numbness over the distal phalanx of the thumb without any residual discoloration when she visited the OPD after discharge on day 15 (Fig 1).
A Rose by any other name
Lupus… it is easier to say lupus than systemic lupus erythematosus, isn’t it? That’s quite a mouthful and I never quite get the spelling right. Lupus means wolf in the great scholarly and equally obscure language called Latin and its relevance is now relegated to astronomy and the occasional medical quiz. Another particularly challenging term is ankylosing spondylitis. It took me at least a year to enunciate this tongue twister without faltering.
I recall a day not too long ago, sitting in a busy sweltering outpatient clinic at Bangalore. The person in front of me was a dove-eyed reflective young girl. She had slightly puffy cheeks, the only physical feature that betrayed her diagnosis: recently diagnosed lupus nephritis stage 5, and discharged on tapering high-dose steroids and mycophenolate. Her parents looked on anxiously as my eyes skimmed through her latest blood and urine tests. The moment I finished my cursory examination, I looked up at them and smiled. There was an audible sigh of relief, a pair of grateful smiles, and glistening eyes. I assembled fragments of the few words of Tamil I knew and managed to say, “She is ok, now.” The young earnest girl looked slightly embarrassed as her parents nudged her and she cleared her throat, and said, “Doctor, I know you explained to me, but my parents didn’t understand, what is the name of my disease?”
I reflexively glanced at my watch, then at the never-ending stack of OPD appointments waiting, and slumped slightly into my chair as the linings of my stomach were self-immolating in an acid bath. I searched the recesses of my brain for some more relevant Tamil fragments and barked brief sentences confabulated with Kannada, Telugu, and Malayalam for good measure. Aided with hand gestures, crude diagrams of kidneys, and an enthusiastic attender of another patient who assumed the role of a questionable translator, we made some progress over the next 5 minutes. Her parents nodded their heads gravely and I marveled at their restraint to keep a straight face. Then her father tapped the girl’s shoulder proudly and spoke haltingly, “My daughter… good English… first class… say English.”
Mercifully, the prodigy spared me another few minutes of this ordeal by assuring me that she understood the charades perfectly well. She pursed her lips together and tried to chant ...es…yel…ee… the syllables repeatedly. I understood that she was struggling and said, “lupus.” Her pupils dilated in surprise, this word was as alien as the singing syllables. They walked away… each of them trying to internalize this new word, this vague Latin snippet that shook their lives and they had no idea what it meant.
At the risk of sounding like a regionalist, instead of whitewashing (rather saffron spraying) history by changing the names of cities and famous railway stations, a better use of our time is to come up with local terms for common diseases. This may seem irrelevant and may never impact your treatment decisions in any way. However, I can’t help but think that, so much is lost in translation, and it might be more palatable to accept a familiar phrase than a foreign jargon.
On a side note, it took me 3 years to master passable Kannada with mildly questionable grammar along with DNB medicine, 2 agonizing years to manage 10 words in Telugu, and I’m engaged in a life time battle with my own mother tongue, Malayalam. Tamil, one of the most expressive and beautiful languages, is my waterloo. Bengali is my nemesis. Before I came to Bangalore, I was considered a linguist who dabbled in English, Hindi, Marathi, Gujarati, and French. And, that ladies and gentleman, is the life of an average doctor in India.
Jai Hind.

Dr.Anu Mohan Desai
DNB Medicine, Fellowship in Rheumatology, St John's National Institute of Health Sciences
Currently pursuing MMED Rheumatology at Wrightington hospital, UK
What was happening in rheumatology
Treatment in rheumatoid arthritis has undergone a sea of change in the past century with hundreds of scientific papers each year exploring the nuances of therapy. Two classic papers selected in this issue signal the advent of new eras in this area with repercussions lasting to this day.
70 years ago: The use of steroids in rheumatoid arthritis
Treatment for rheumatoid arthritis was scarce, and most patients eventually developed deformity. This study announced a completely new treatment for RA: corticosteroids. The study talks about the background of the development of “Compound E” – Adrenocorticotropic hormone (ACTH) from a pituitary extract and its use in patients. Compound E was administered intramuscularly for a brief period of 1 month. The study had a small sample size of only 14 patients, all with severe disease and most were wheelchair bound or bedridden. All patients had a dramatic clinical response with a reduction in the number of swollen and tender joints; a few regained their ability, which was virtually unheard of before in patients with this crippling disease. Since it was a short-term trial and not well controlled, adverse drug reactions were rare, although hirsutism and edema were reported.
This study heralded the advent of the use of corticosteroids in rheumatoid arthritis in particular and indeed autoimmune diseases, in general. Practical difficulties were yet to be conquered: extremely small amounts of compound E could be synthesized. The paper itself published a caveat: that significant amounts of corticosteroids were unlikely to be available even for future clinical research for 3 years! Needless to say, these obstacles were later surpassed and steroids are in widespread use until this day. This work, followed by subsequent research, led Dr. Hench to win the Nobel Prize for physiology or medicine, the only rheumatologist to have won one.
Hench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydro-corticosterone-compound E) and of pituitary adreno-corticotropic hormone on rheumatoid arthritis – preliminary report. Proceedings of the Staff Meetings of the Mayo Clinic 1949; 24:181–197
20 years ago: Cytokine inhibition in RA
This was the very first time a monoclonal antibody to tumor necrosis factor (TNF) was used in a clinical trial. The authors conducted a randomized double-blind trial to compare a single infusion of two doses of 1 and 10 mg/kg cA2 with placebo in 73 patients with active rheumatoid arthritis (RA). A Paulus 20% response at 4 weeks was the primary endpoint. Nineteen of 24 patients treated with high-dose therapy responded, compared with 11 in the low-dose group and 4 in the placebo group, thus providing neat, dose-dependent proof that the blockade of specific inflammatory cytokines was effective in RA. Significant reductions in swollen and tender joint counts, pain and overall disease activity, erythrocyte sedimentation rate (ESR), and c-reactive protein (CRP) were documented. Both clinical and laboratory parameters improved in the first week itself, and remained low in the high-dose group over a period of 4 weeks. Side effects were noted: most were minor, although one patient developed a serious respiratory infection.
Apart from providing the community a new and effective drug, this work presented compelling clinical evidence that chimeric tumor necrosis alpha plays a significant role in inflammation in RA. This has had many subsequent ramifications in blocking other cytokines and therefore signaling the advent of a new era of using biological disease-modifying antirheumatic drugs (DMARDs), and they are an important part of an overall treatment plan (DMARDs) in RA.
Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS, Leeb B, Breedveld FC, Macfarlane JD, Bijl H, Woody JN. Randomized double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet 1994; 344:1105–1110

Dr. Sanat Pathak, MD DM
Asst Prof, Dept of medicine, BJ Government Medical College, Pune
From the bench
Human neonatal thymectomy induces altered B-cell responses and autoreactivity
Van den Broek T, Madi A, Delemarre EM et al. Eur J Immunol 2017 Nov 47(11) : 1970-81.
An association between T-cell lymphopenia and autoimmunity has long been proposed, but it remains to be elucidated whether T-cell lymphopenia affects B-cell responses to autoantigens. Human neonatal thymectomy (Tx) results in a decrease in T-cell numbers, and we used this model to study the development of autoreactivity. Two cohorts of neonatally thymectomized individuals were examined, a cohort of young (1–5 years post-Tx, n = 10–27) and older (>10 years, n = 26) children, and compared with healthy age-matched control subjects. T-cell and B-cell subsets were assessed and autoantibody profiling was performed. Early post-Tx, a decrease in T-cell numbers (2.75 × 109/L vs 0.71 × 109/L) and an increased proportion of memory T cells (19.72 vs 57.43%) were observed. The presence of autoantibodies was correlated with an increased proportion of memory T cells in thymectomized children. No differences were seen in percentages of different B-cell subsets between the groups. The autoantigen microarray showed a skewed autoantibody response after Tx. In the cohort of older individuals, autoantibodies were present in 62% of the thymectomized children, while they were found in only 33% of the healthy control subjects. Overall, our data suggest that neonatal Tx skews the autoantibody profile. Preferential expansion and preservation of Treg (regulatory T) cell stability and function may contribute in preventing autoimmune disease development after Tx.
Transcription Factor IRF4 Promotes CD8+ T Cell
Exhaustion and Limits the Development of Memory like T Cells during Chronic Infection
Man K, Gabriel SS, Liao Y et al. Immunity 2017 Dec 19, 47(6);1129-1141.
During chronic stimulation, CD8+ T cells acquire an exhausted phenotype characterized by expression of inhibitory receptors, down-modulation of effector
function, and metabolic impairments. T-cell exhaustion protects from excessive immunopathology but limits clearance of virus-infected or tumor cells.
We
transcriptionally profiled antigen-specific T cells from mice infected with lymphocytic choriomeningitis virus strains that cause acute or chronic disease. T-cell exhaustion during chronic infection was driven by high amounts of T-cell receptor (TCR)-induced transcription factors IRF4, BATF, and NFATc1. These regulators promoted expression of inhibitory receptors, including PD-1, and mediated impaired
cellular metabolism. Furthermore, they repressed the expression of TCF1, a transcription factor required for memory T-cell differentiation.
Reducing
IRF4 expression restored the functional and metabolic properties of antigen-specific T cells and promoted memory-like T-cell development. These findings indicate that IRF4 functions as a central node in a TCR-responsive transcriptional circuit
that establishes and sustains T-cell exhaustion during chronic infection.
Genome-Wide Association Meta-Analysis Reveals Novel Juvenile Idiopathic Arthritis Susceptibility Loci
McIntosh LA, Marion MC, Sudman M et al. Arthritis Rheumatol 2017 Nov ; 69(11) 2222-2232.
Objective
Juvenile idiopathic arthritis (JIA) is the most common childhood rheumatic disease and has a strong genomic component. To date, JIA genetic association studies have had limited sample sizes, used heterogeneous patient populations, or included only candidate regions. The aim of this study was to identify new associations between JIA patients with oligoarticular disease and those with IgM rheumatoid factor (RF)−negative polyarticular disease, which are clinically similar and the most prevalent JIA disease subtypes.
Methods
Three cohorts comprising 2751 patients with oligoarticular or RF-negative polyarticular JIA were genotyped using the Affymetrix Genome-Wide SNP Array 6.0 or the Illumina HumanCoreExome-12+ Array. Overall, 15,886 local and out-of-study controls, typed on these platforms or the Illumina HumanOmni2.5, were used for association analyses. High-quality single-nucleotide polymorphisms (SNPs) were used for imputation to 1000 Genomes prior to SNP association analysis.
Results
Meta-analysis showed evidence of association (P < 1 × 10−6) at 9 regions: PRR9_LOR (P = 5.12 × 10−8), ILDR1_CD86 (P = 6.73 × 10−8), WDFY4 (P = 1.79 × 10−7), PTH1R (P = 1.87 × 10−7), RNF215 (P = 3.09 × 10−7), AHI1_LINC00271 (P = 3.48 × 10−7), JAK1 (P = 4.18 × 10−7), LINC00951 (P = 5.80 × 10−7), and HBP1 (P = 7.29 × 10−7). Of these, PRR9_LOR, ILDR1_CD86, RNF215, LINC00951, and HBP1 were shown, for the first time, to be autoimmune disease susceptibility loci. Furthermore, associated SNPs included cis expression quantitative trait loci for WDFY4, CCDC12, MTP18, SF3A1, AHI1, COG5, HBP1, and GPR22.
Conclusion
This study provides evidence of both unique JIA risk loci and risk loci overlapping between JIA and other autoimmune diseases. These newly associated SNPs are shown to influence gene expression, and their bounding regions tie into molecular pathways of immunologic relevance. Thus, they likely represent regions that contribute to the pathology of oligoarticular JIA and RF-negative polyarticular JIA.
An analysis of IL-36 signature genes and individuals with IL1RL2 knockout mutations validates IL-36 as a psoriasis therapeutic target
Satveer K. Mahil, Marika Catapano, Paola Di Meglio, Nick Dand et al. Sci Transl Med. 2017 Oct (11):9 (411)
Interleukin (IL)–36a, IL-36b, and IL-36g are innate mediators of acute epithelial inflammation. We sought to demonstrate that these cytokines are also required for the pathogenesis of plaque psoriasis, a common and chronic skin disorder, caused by abnormal T helper 17 (TH17) cell activation. To investigate this possibility, we first defined the genes that are induced by IL-36 cytokines in primary human keratinocytes. This enabled us to demonstrate a significant IL-36 signature among the transcripts that are upregulated in plaque psoriasis and the susceptibility loci associated with the disease in genome-wide studies. Next, we investigated the impact of in vivo and ex vivo IL-36 receptor blockade using a neutralizing antibody or a recombinant antagonist. Both inhibitors had marked anti-inflammatory effects on psoriatic skin, demonstrated by statistically significant reductions in IL-17 expression, keratinocyte activation, and leukocyte infiltration. Finally, we explored the potential safety profile associated with IL-36 blockade by phenotyping 12 individuals carrying knockout mutations of the IL-36 receptor gene. We found that normal immune function was broadly preserved in these individuals, suggesting that IL-36 signaling inhibition would not substantially compromise host defenses. These observations, which integrate the results of transcriptomics and model system analysis, pave the way for early-stage clinical trials of IL-36 antagonists.
Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis
David Lagares, Alba Santos, Paula E. Grasberger, Fei Liu, Clemens K. Probst et al. Sci Transl Med. 2017 Dec 13;9(420)
Persistent myofibroblast activation distinguishes pathological fibrosis from physiological wound healing, suggesting that therapies selectively inducing myofibroblast apoptosis could prevent progression and potentially reverse established fibrosis in diseases such as scleroderma, a heterogeneous autoimmune disease characterized by multiorgan fibrosis. We demonstrate that fibroblast-to-myofibroblast differentiation driven by matrix stiffness increases the mitochondrial priming (proximity to the apoptotic threshold) of these activated cells.
Mitochondria in activated myofibroblasts, but not quiescent fibroblasts, are primed by death signals such as the proapoptotic BH3-only protein BIM, which creates a requirement for tonic expression of the antiapoptotic protein BCL-XL to sequester BIM and ensure myofibroblast survival. Myofibroblasts become particularly susceptible to apoptosis induced by “BH3 mimetic” drugs inhibiting BCL-XL such as ABT-263. ABT-263 displaces BCL-XL binding to BIM, allowing BIM to activate apoptosis on stiffness-primed myofibroblasts. Therapeutic blockade of BCL-XL with ABT-263 (navitoclax) effectively treats established fibrosis in a mouse model of scleroderma dermal fibrosis by inducing myofibroblast apoptosis. Using a BH3 profiling assay to assess mitochondrial priming in dermal fibroblasts derived from patients with scleroderma, we demonstrate that the extent of apoptosis induced by BH3 mimetic drugs correlates with the extent of their mitochondrial priming, indicating that BH3 profiling could predict apoptotic responses of fibroblasts to BH3 mimetic drugs in patients with scleroderma. Together, our findings elucidate the potential efficacy of targeting myofibroblast antiapoptotic proteins with BH3 mimetic drugs in scleroderma and other fibrotic diseases.
Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis
Xavier Bossuyt et al, Nature reviews rheumatology Nov 2017.
Anti-neutrophil cytoplasmic antibodies (ANCAs) are valuable laboratory markers used for the diagnosis of well-defined types of small-vessel vasculitis, including granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). According to the 1999 international consensus on ANCA testing, indirect immunofluorescence (IIF) should be used to screen for ANCAs, and samples containing ANCAs should then be tested by immunoassays for proteinase 3 (PR3)-ANCAs and myeloperoxidase (MPO)-ANCAs. The distinction between PR3-ANCAs and MPO-ANCAs has important clinical and pathogenic implications. As dependable immunoassays for PR3-ANCAs and MPO-ANCAs have become broadly available, there is an increasing international agreement that high-quality immunoassays are the preferred screening method for the diagnosis of ANCA-associated vasculitis. The present Consensus Statement proposes that high-quality immunoassays can be used as the primary screening method for patients suspected of having the ANCA-associated vasculitides GPA and MPA without the categorical need for IIF, and presents and discusses evidence to support this recommendation.

Dr. Avinash Jain, DM Resident, SGPGIMS Lucknow

Rutwiz Mistry, SR, DM Resident, SGPGIMS Lucknow
Puzzle of the issue
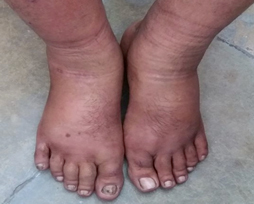
Pitting dilemmas!
A 45-year-old auto-rickshaw driver presented with bilateral symmetrical painful swelling of hands and feet for the past 6 months. The swelling was pitting in nature. On examination, the patient was vitally stable with normal systemic examination.
He was treated elsewhere with multiple courses of Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids with a working diagnosis of Remitting seronegative symmetrical synovitis with pitting edema (RS3PE) but failed to respond.
Investigations
Hb: 10.1 mg/dL,
TC: 8700,
DC: 80/15/3/2/0,
platelet: 2.4 lacs.,
ESR: 45 mm,
CRP: negative.
Total protein: 5.4, albumin: 2.2
RF/ACCP/ANA: Negative
X-ray chest: Normal
Urine R/M: Grossly normal
Renal, cardiac, and hepatic causes were ruled out.
Leprosy was ruled out.
What can be the diagnosis?
(mail your answers to dr.pujasrivastava@gmail.com – names and designations of fellows with correct answers will be mentioned in the next issue newsletter and suitably awarded at the IRACON by the editorial team of NL)

Dr. Puja Srivastava Consultant Rheumatologist
VS Hospital & NHL Med College
& STAR Clinics, Ahmedabad

Dr. Nibha Jain, Fellow, Rheumatology
VS Hospital & NHL Municipal Med College, Ahmedabad