Fellows’ corner

Dr Shefali Sharma, Rheumatology, PGIMER, Chandigarh
Sanket Shah, Dr. Chengappa KG, Dr. V.S. Negi, Clinical Immunology and Rheumatology, JIPMER, Pondicherry
CLINICAL CASE
A 47-year-old gentleman from Tamil Nadu, involved in agricultural work, presented to the rheumatology department with the manifestation of inflammatory symmetrical polyarthritis, involving small and large joints of upper limbs and lower limbs, which started 2 months back, and was associated with myalgia and fatigue. Twenty days prior to the onset of arthritis, the patient had a high-grade fever associated with chills and dysuria with a borderline rise in serum creatinine (1.6 mg/dl). He received treatment on the lines of urinary tract infection with intravenous antibiotics, with only partial relief in complaints. Twenty days following the onset of arthritis, he developed redness of the right eye with minimal pain. He was diagnosed with episcleritis by an ophthalmologist. Besides this, he had had a weight loss of 5 kg over the last 2 months. On examination at the time of presentation, he had sinus tachycardia and fever and a normal blood pressure. General physical examination showed pallor and generalized lymphadenopathy, with the largest lymph node in the axillary region being 2×3 cm2. Musculoskeletal examination suggested tenderness, involving bilateral MCPs, PIPs, DIPs, wrists, shoulders, knees, and ankles. The pain and tenderness in his joints were disproportionate to swelling. Systemic examination did not reveal hepatosplenomegaly or ascites, and cardiorespiratory examination was unremarkable. Neurological examination did not reveal any proximal weakness or any other deficit.
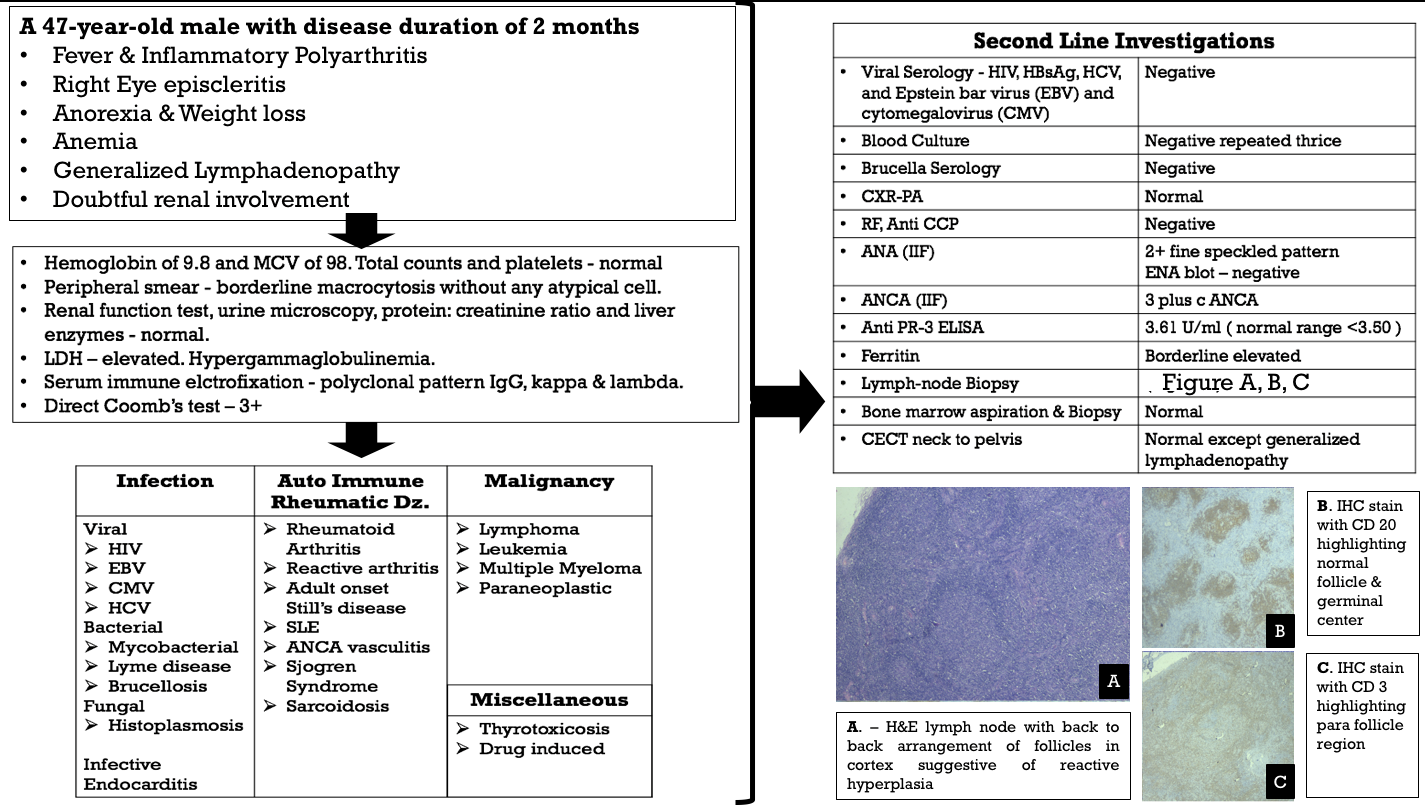
Workup on the patient has been summarized in an image below.
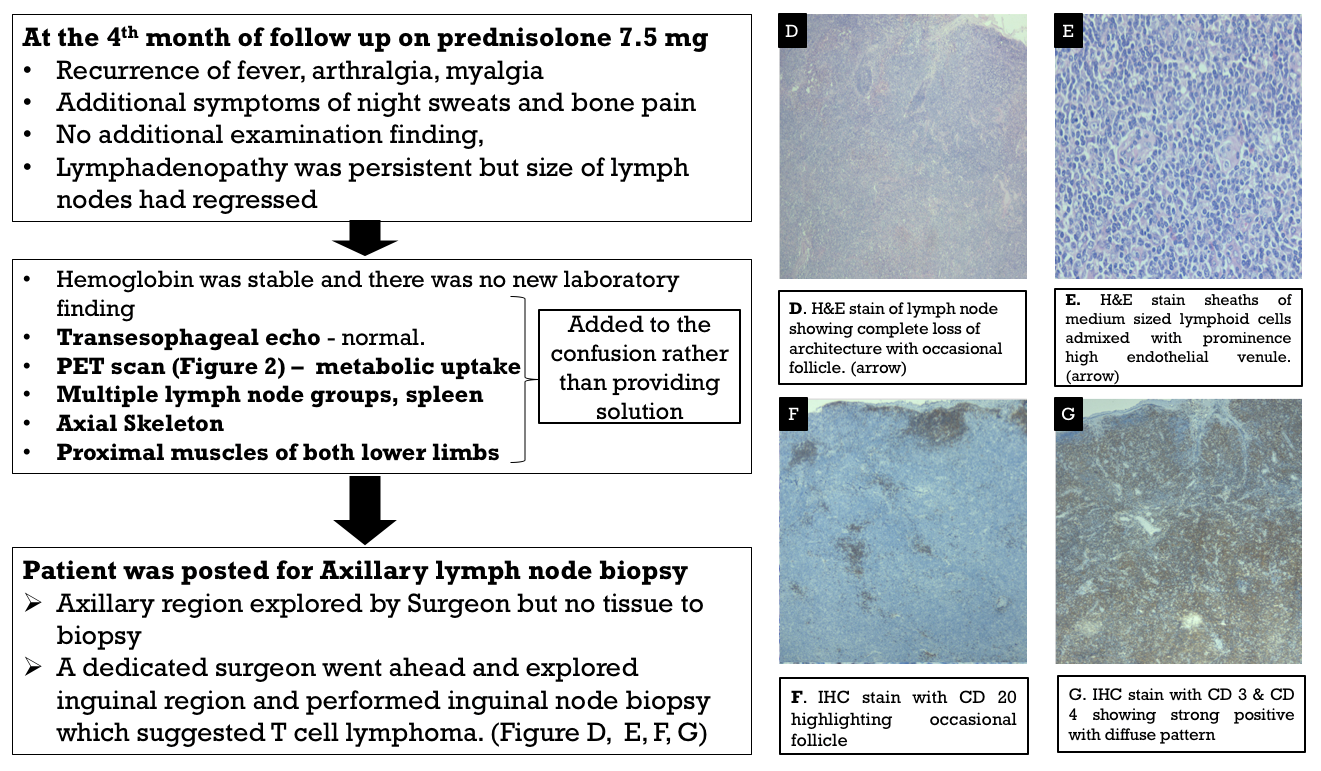
With the tissue diagnosis of lymphoma now, he was referred to the oncology department for further management.
DISCUSSION
Autoimmune rheumatic manifestations are commonly described in B-cell lymphomas, although the literature on the same related to T-cell lymphoma (TCL) is limited to individual case reports and small case studies. Peripheral TCL-not otherwise specified (PTCL-NOS), as diagnosed in this case, is the most common of TCLs, followed by anaplastic, angioimmunoblastic, and extra-nodal NK/TCL, which present with various autoimmune rheumatological manifestations, including autoimmune hemolytic anemia, thrombocytopenia, polyarthritis, episcleritis, erythroderma, psoriasis, and pemphigus.1 As described in literature, male gender, older age, an absence of erosions on X-rays, explosive onset of arthritis, and the presence of atypical features, such as generalized lymphadenopathy and prominent constitutional symptoms, point toward an underlying malignancy.2 The learning point in this case was the surgeon’s decision to remove the other lymph node rather than sending the patient back, which is not uncommon in real practice.
CONCLUSION
Super specialties are all about linking the subtleties to form a diagnostic matrix. Rheumatology, more than any other subspecialty of medicine, forces the clinician to explore varied possibilities that can mimic autoimmune diseases. In fact, multiple test positivities only add to the confusion. The crux lies in a thorough clinical evaluation and being open to a wide range of differential diagnoses when all the lines of evidence gathered do not fit into a single one. Confidence in clinical judgement, insistence on reaching a diagnosis, and team work work well for both the patient and the care-giver.
REFERENCES
- Van den Bergh M, Alvarez-Argote J, Panwala AH, et al. Autoimmune disorders in patients with T-cell lymphoma: a comprehensive review. Curr Med Res Opin. 2015;31(10):1861–1870.
- Dabrowska-Zimoń A, Brzosko M. [A review of paraneoplastic rheumatic syndromes]. Ann Acad Med Stetin. 2006;52 Suppl 2:17–22. Review.

Dr.Sanket Shah, 2nd year DM, Clinical Immunology and Rheumatology, JIPMER
Fellows’ corner: From the bench
Lupus antibodies induce behavioral changes mediated by microglia and blocked by ACE inhibitors.
Nestor J, et al. J Exp Med. 2018.Cognitive impairment occurs in 40%–90% of patients with systemic lupus erythematosus (SLE), which is characterized by auto-antibodies to nuclear antigens, especially DNA. We discovered that a subset of anti-DNA antibodies, termed DNRAbs, cross-reacts with the N-methyl-d-aspartate receptor (NMDAR) and enhances NMDAR signaling. In patients, the presence of DNRAb associates with spatial memory impairment. In a mouse model, DNRAb-mediated brain pathology proceeds through an acute phase of excitotoxic neuron loss, followed by persistent alteration in neuronal integrity and spatial memory impairment. The latter pathology becomes evident only after DNRAbs are no longer detectable in the brain. Here we investigate the mechanism of long-term neuronal dysfunction mediated by transient exposure to antibody. We show that activated microglia and C1q are critical mediators of neuronal damage. We further show that centrally acting inhibitors of angiotensin-converting enzyme (ACE) can prevent microglial activation and preserve neuronal function and cognitive performance. Thus, ACE inhibition represents a strong candidate for clinical trials aimed at mitigating cognitive dysfunction.
Targeting the TLR4—MD2 axis in systemic sclerosis.
Swati Bhattacharyya, Hang Yin, John Varga. JCI Insight. 2018Persistent fibrosis in multiple organs is the hallmark of systemic sclerosis (SSc). Recent genetic and genomic studies implicate that TLRs and their damage-associated molecular pattern (DAMP) are endogenous ligands in fibrosis. To test the hypothesis that TLR4 and its co-receptor myeloid differentiation 2 (MD2) drive fibrosis persistence, we measured MD2/TLR4 signaling in tissues from patients with fibrotic SSc, and we examined the impact of MD2 targeting using a potentially novel small molecule. Levels of MD2 and TLR4, and a TLR4-responsive gene signature, were enhanced in SSc skin biopsies. We developed a small molecule that selectively blocks MD2, which is uniquely required for TLR4 signaling. Targeting MD2/TLR4 abrogated inducible and constitutive myofibroblast transformation and matrix remodeling in fibroblast monolayers, as well as in three-dimensional scleroderma skin equivalents and human skin explants. Moreover, the selective TLR4 inhibitor prevented organ fibrosis in several preclinical disease models and mouse strains, besides reversed pre-existing fibrosis. Fibroblast-specific deletion of TLR4 in mice afforded a substantial protection from skin and lung fibrosis. By comparing experimentally generated fibroblast TLR4 gene signatures with SSc skin biopsy gene expression data sets, we identified a subset of SSc patients displaying an activated TLR4 signature. Together, results from these human and mouse studies implicate MD2/TLR4-dependent fibroblast activation as a key driver of persistent organ fibrosis. The results suggest that SSc patients with high TLR4 activity might show an optimal therapeutic response to selective inhibitors of MD2/TLR4 complex formation.
Modulation of Inflammatory Arthritis in Mice by Gut Microbiota Through Mucosal Inflammation and Autoantibody Generation
Widian K. Jubair A and R, Aug 2018Objective
Observations of microbial dysbiosis in patients with rheumatoid arthritis (RA) have raised an interest in studying microbial–mucosal interactions as a potential trigger of RA. Using the murine collagen-induced arthritis (CIA) model, we conducted this study to test our hypothesis that microbiota modulate immune responses leading to autoimmune arthritis.
MethodsCIA was induced by immunization of mice with type II collagen (CII) in adjuvant on days 0 and 21, with arthritis appearing on days 23 and 24. Intestinal microbiota were profiled by 16S ribosomal RNA sequencing every 7 days during the course of CIA, and intestinal mucosal changes were evaluated on days 14 and 35. Then, microbiota were depleted either early (7 days before immunization) or late (day 21 after immunization) by the administration of broad-spectrum antibiotics. Disease severity; autoantibody and systemic cytokine production; and intestinal mucosal responses were monitored in the setting of microbial reduction.
ResultsSignificant dysbiosis and mucosal inflammation occurred early in CIA, prior to visible arthritis, and continued to evolve during the course of disease. Depletion of the microbiota prior to the induction of CIA resulted in an ~40% reduction in disease severity and in significantly reduced levels of serum inflammatory cytokines and anti-CII antibodies. In intestinal tissue, production of interleukin (IL)-17A and IL-22 was delayed. Unexpectedly, microbial depletion during the late phase of CIA resulted in a >50% decrease in disease severity. Anti-CII antibodies were mildly reduced but were significantly impaired in their ability to activate complement, likely due to altered glycosylation profiles.
ConclusionThese data support a model in which intestinal dysbiosis triggers mucosal immune responses that stimulate T and B cells, which are key for the development of inflammatory arthritis.
Insufficient IL-10 Production as a Mechanism Underlying the Pathogenesis of Systemic Juvenile Idiopathic Arthritis
Maya Imbrechts et al. J Immunol. Sept 2018.Systemic juvenile idiopathic arthritis (sJIA) is a childhood-onset immune disorder of unknown cause. One of the concepts is that the disease results from an inappropriate control of immune responses to an initially harmless trigger. In the current study, we investigated whether sJIA may be caused by defects in IL-10—a key cytokine in controlling inflammation. We used a translational approach, with an sJIA-like mouse model and sJIA patient samples. The sJIA mouse model relies on injection of CFA in IFN-γ-deficient BALB/c mice; corresponding wild-type (WT) mice only develop a subtle and transient inflammatory reaction. Diseased IFN-γ-deficient mice showed a defective IL-10 production in CD4+ regulatory T cells, CD19+ B cells, and CD3−CD122+CD49b+ NK cells, with B cells as the major source of IL-10. In addition, neutralization of IL-10 in WT mice resulted in a chronic immune-inflammatory disorder clinically and hematologically reminiscent of sJIA. In sJIA patients, IL-10 plasma levels were strikingly low as compared with proinflammatory mediators. Furthermore, CD19+ B cells from sJIA patients showed a decreased IL-10 production, after both ex vivo and in vitro stimulations. In conclusion, IL-10 neutralization in CFA-challenged WT mice converts a transient inflammatory reaction into a chronic disease and represents an alternative model for sJIA in IFN-γ-competent mice. Cell-specific IL-10 defects were observed in sJIA mice and patients, together with an insufficient IL-10 production to counterbalance their proinflammatory cytokines. Our data indicate that a defective IL-10 production contributes to the pathogenesis of sJIA.
A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism
Eleonora Trotta, Nat Med., July 2018Interleukin-2 has been shown to suppress immune pathologies by preferentially expanding regulatory T cells (Tregs). However, this therapy has been limited by off-target complications due to pathogenic cell expansion. Recent efforts have been focused on developing a more selective IL-2. It is well documented that certain anti-mouse IL-2 antibodies induce conformational changes that result in selective targeting of Tregs. We report the generation of a fully human anti-IL-2 antibody, F5111.2, that stabilizes IL-2 in a conformation that results in the preferential STAT5 phosphorylation of Tregs in vitro and selective expansion of Tregs in vivo. When complexed with human IL-2, F5111.2 induced remission of type 1 diabetes in the NOD mouse model, reduced disease severity in a model of experimental autoimmune encephalomyelitis, and protected mice against xenogeneic graft-vs.-host disease. These results suggest that IL-2-F5111.2 may provide an immunotherapy to treat autoimmune diseases and graft-vs.-host disease.

Dr. Avinash Jain, DM, Clinical Immunology and Rheumatology, SGPGIMS
Scenes From a Rheumatology Conference: The Usual Suspects
Venue: A conference center of a five-star hotel.
The biggest subconscious incentive for attending the event as it is the only legitimate means of entry for 90% of the attendees. The perfumed air, mellow lighting, exotic flowers, sparkling toilets, expensive upholstery, and the promise of a delicious buffet meal. And the after party…ahem.
Attendees of a conference are segregated into two groups: Delegates and organizers; however, this is an oversimplification. I propose a much more comprehensive classification, as follows:
The Super Antigen: The Chairperson/Chief Organizer/Secretary of this event. He/She has perilously balanced clinical commitments with the exacting demands of organizing the extravaganza. Sleep-deprived and starved, yet running at supreme efficiency (?? Steroids), he/she shudders at the sight of a cloud yet has formulated a contingency plan for Armageddon. His/her worst fear is a tsunami, or worse, a speaker who fails to arrive.
The Apoptotic Cell: The speaker who was part of the original draft, but had to regretfully cancel at some point due to an inconvenient tragedy. He/She will be hastily replaced by another speaker of equal caliber and eminence, who feels compelled to announce this unknown arrangement as a disclaimer before presenting his/her last-minute marvel.
The Overexpressed Cytokine: The most prominent sighting of this individual is during the Q and A session after an expert’s session. The panelists have cast the speaker to the lions, and the most aggressive and vitriolic member of the audience exploits this opportunity to the fullest. Peace prevails only when the chairperson steps in and announces time constraints.
The Downregulated Receptor: The shy member of the audience who was reluctant to ask his/her doubt, fearing exposure of ignorance and ridicule. Usually a bewildered fresh graduate. A regrettable waste of a tantalizing opportunity!
The Phagocyte: No prizes for guessing, the buffet table champion, who can expertly balance heaps on a single plate and manage multiple servings before food runs out.
The Co-stimulators: Close colleagues, who rarely have the opportunity to interact due to their chaotic lives, find solace in each other’s company during the event. They can be spotted huddled in a corner, discussing challenging clinical cases and their children’s latest academic exploits with equal fervor.
The Rapid Sequencers: Thanks to free WiFi, there is an enthusiastic propaganda that has been running parallel to the proceedings. Group selfies with conference banners in the background, or blurry photos of presenters at the podium captured by their friends as promised, find their way on Facebook, WhatsApp groups, and Twitter. Ah, the boon and banality of modern life.
Despite unreliable microphones, flickering lights, and the invariable annoying ring tone that pierces the solemnity of a lecture (due to non-adherence of basic housekeeping rules), the event is a success. The attendees are dazzled with volatile information while earning much needed credit points and the organizers can finally relax.
We have dipped our feet in the ocean called Rheumatology. Until the next meeting…

Dr. Anu Desai, pursuing MMED Rheumatology at Wrightington Hospital, UK
What was Happening in Immunology 25 Years Ago:
The Discovery of Checkpoints in Lymphocytes and Their TargetingTasuku Honjo, Professor at Kyoto University, and his group explored programmed cell death in lymphocytes. They identified a new gene that was found to code for a PD1 protein: A novel immunoglobulin receptor which they called PD-1. They found that this gene and protein were activated in those cells destined for programmed cell death. In other papers, the group investigated the role of PD-1. If the PD-1 gene was disrupted, there was lymphocyte proliferation and autoimmunity; the experimental animals developed a lupus-like illness. Thus, they demonstrated the function of PD-1 as a lymphocyte, and particularly T cell, “brake”. Nearly our entire knowledge of the PD-1 molecular brake comes from this pioneering work.
Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895.
Nishimura H, Nose M, Hiai H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151.
James P Allison, at UC Berkeley, worked on a second similar receptor: CTLA-4. He was among the investigators who had previously demonstrated that CTLA-4 too works as a molecular brake on T cells. The usage of augmenting CTLA-4 activity in the treatment of autoimmune disease was already being worked upon: We were to derive abatacept from this work in the treatment of RA. The idea of harnessing one’s own immunity to induce an attack against cancer cells had been previously thought of, and attempts had been made—such as introducing concomitant bacterial infection to activate the system—and these met with limited success. James Allison pioneered the concept of removing the “brakes” from immune cells, which might make them combat cancer cells. He developed an antibody against CTLA-4. In vivo administration of these antibodies in animals resulted in the rejection of tumors, including pre-established tumors. Furthermore, this rejection resulted in an immunity to a secondary exposure to tumor cells. After this initial breakthrough and despite lukewarm responses from the pharmaceutical industry, he followed up his discovery till it came from bedside to bench as an anticancer strategy in humans.
Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996; 271(5256):1734–1736.
Both PD-1 and CTLA-4, brakes or checkpoints that work by different mechanisms, have been targeted successfully in cancer therapy. In 2010, a study showed striking effects in advanced melanoma, and the repertoire has grown to include many types of metastatic cancers. In the limited armamentarium of drugs against advanced cancers, this was an important addition. For these discoveries, Allison and Honjo shared this year’s Nobel Prize in Physiology and Medicine.

Dr. Sanat Pathak, MD DM
Asst Prof, Dept of medicine, BJ Government Medical College, Pune
Puzzle of the Issue: Last Issue’s Answer
Diagnosis: Primary Sjögren’s with Lymphocytic Interstitial Pneumonitis (LIP)
Lymphocytic interstitial pneumonitis is a benign lymphoproliferative disorder in Sjögren’s syndrome. It is characterized by a diffused proliferation of polyclonal lymphocytes and plasma cells in the pulmonary parenchymal interstitium, with lymphoid follicles and germinal centers (non-lymphomatous pulmonary lymphoid disorders). In a cohort of patients with LIP, 28% had Sjögren’s syndrome.1 It is also associated with AIDS, autoimmune thyroid disease, SLE, Castleman disease, common variable immune deficiency, RA, and pulmonary amyloidosis.
Most patients have respiratory symptoms (particularly dyspnea and cough), with bilateral inspiratory crackles. Classically, PFT shows a restrictive syndrome depending on the progression of the disease.
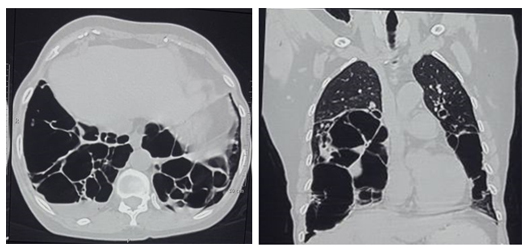
The most frequent abnormalities shown on CT scans are thickened bronchovascular bundles (evoking an association with follicular bronchiolitis), nodules, ground-glass opacities, and thickening of interlobular septa. Studies have reported cysts in 68%–82% of patients with LIP.2 Surgical lung biopsy and assessment of clonality are essential to exclude the diagnosis of lymphoma.
Lymphocytic interstitial pneumonitis seems to be a reversible lung disease with a potential risk of progression. The majority of patients treated with corticosteroids remain clinically stable or show improvement.3 Other immunosuppressive agents (azathioprine, cyclophosphamide, and chlorambucil) have been used but with variable response. Rituximab has been described as effective in one patient.
References
1. Liebow AA, Carrington C. Diffuse pulmonary lymphoreticular infiltrations associated with dysproteinemia. Med Clin North Am. 1973;57:809–843.
2. Swigris JJ, Berry GJ, Raffin TA, et al. Lymphoid interstitial pneumonia: A narrative review. Chest. 2002;122:2150–2164.
3. Johkoh T, Ichikado K, Akira M, et al. Lymphocytic interstitial pneumonia: Follow-up CT findings in 14 patients. J Thorac Imaging. 2000;15:162–167.
Puzzle of the Month: October 2018
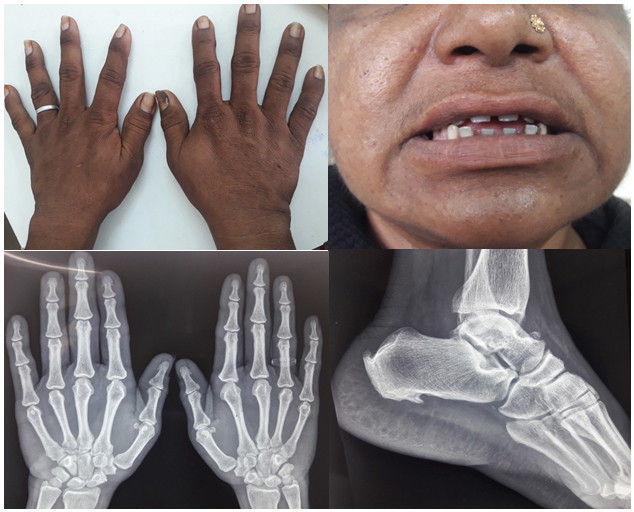
A middle-aged lady presented with 1-year duration of arthralgias involving large peripheral joints (shoulder, knee, and elbows) associated with bilateral carpal tunnel syndrome. No h/o sicca symptoms. She also had lower back pain more in lumbar area without early morning stiffness. She noticed tightening of clothes over a period of 1 year, with an increase in ring and shoe size. She had oily skin with hirsutism and acanthosis nigricans. Her baseline investigations (CBC, creatinine, LFT) and inflammatory markers (ESR:20, CRP: 1 mg/l) were within normal limits.
What is the most likely cause of her symptoms?

Dr. Puja Srivastava Consultant Rheumatologist
VS Hospital & NHL Med College
& STAR Clinics, Ahmedabad

Dr. Nibha Jain, Fellow, Rheumatology
VS Hospital & NHL Municipal Med College, Ahmedabad
Winners will be suitably rewarded!